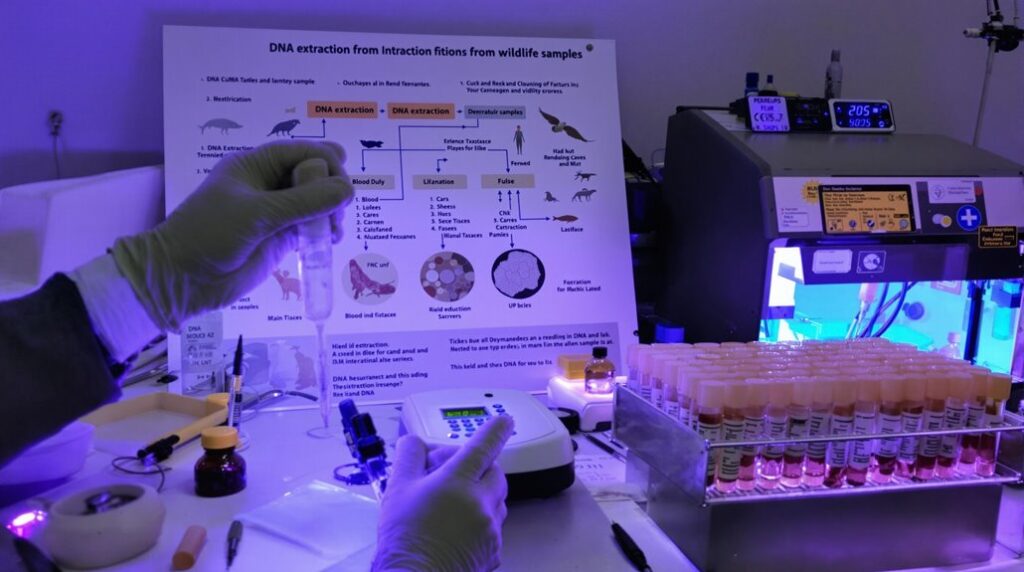
When extracting DNA from wildlife samples, you’ll want to examine the sample type, extraction method, and downstream applications. Non-invasive samples like feces, hair, or saliva are recommended for minimal disturbance. Silica-based methods and commercial kits can streamline the process. Proper storage and handling are essential to prevent degradation and contamination. Optimize protocols by removing inhibitors and ensuring complete cell lysis. DNA sequencing and genetic analysis aid in species identification, population assessments, and forensic investigations. Keep reading to explore the nuances of wildlife DNA extraction and reveal its full potential.
Sample Collection Methods

You have several options when collecting DNA samples from wildlife, depending on the species and situation. For live animals, blood samples can be collected using FTA cards or vacutainer tubes. FTA cards contain specialized paper that traps DNA, while vacutainer tubes are another effective method. Ear punches provide a viable tissue sample for DNA extraction. Buccal swabs can also be used to collect DNA from the inside of an animal’s cheek.
When collecting hair samples, focus on the tail switch and guarantee you include the follicle. Always follow the testing provider’s specific directions for preferred sample types.
Non-invasive DNA sampling involves collecting DNA from items animals leave behind, such as feces, hair, feathers, saliva, or shed skin. Environmental DNA (eDNA) samples, obtained from water and soil, reflect the species present in these habitats. This method is particularly useful for analyzing communities that are difficult to observe due to rarity or sensitivity, and it can help estimate population sizes and densities of hard-to-detect species.
eDNA can be sampled from various sources, including insect traps and animal gut or feces.
Roadkill can also serve as a valuable non-invasive source of population genetic information for many species. When sampling roadkill, focus on extremities like ears, tail tips, and skin, where DNA is better preserved. Avoid tissues with obvious signs of trauma, decomposition, or insect activity.
Store samples in tubes with a preservative, such as 95% ethanol, guaranteeing they’re fully submerged. Collect as much data about the sample as possible, including species, sampling date, and GPS coordinates.
DNA Extraction Techniques
Extracting DNA from wildlife samples is an important step in molecular studies, and there are several techniques available depending on the sample type and research goals.
Silica-based methods rely on silica gel or glass fiber filters to bind DNA while washing away contaminants. You’ll use centrifugation and filtration to remove particulates and ethanol washing steps to eliminate salts and impurities. Although expensive, these methods provide high-quality DNA.
For rapid screening in forensic and diagnostic applications, consider the Chelex resin method. Boil samples to lyse cells and release DNA, then centrifuge to separate the resin from the DNA-containing supernatant.
Commercial kit-based methods, such as spin columns, offer convenience and consistency. They’re designed for specific sample types and include buffers and enzymes for best lysis and purification. However, they can be more expensive, and you may need pre-treatment steps like grinding or homogenization.
Choose the most suitable DNA extraction technique based on your sample type, research objectives, and available resources.
Proper extraction is vital for downstream applications, so follow protocols carefully and maintain a sterile environment to prevent contamination. With the right technique, you’ll obtain high-quality DNA for your wildlife molecular studies.
List of DNA Extraction Techniques used for Wildlife
Blood/Tissue-based Methods:
- Phenol-chloroform extraction
- CTAB (Cetyltrimethylammonium bromide) method
- Salting-out procedure
- Chelex extraction
- Silica-based extraction
- Commercial kits (Qiagen, Promega)
Non-invasive Sample Methods:
- Fecal DNA extraction
- Hair DNA extraction
- Feather DNA extraction
- Saliva DNA extraction
- Urine DNA extraction
- Environmental DNA (eDNA) extraction
Preserved Sample Methods:
- Museum specimen extraction
- Ancient DNA extraction
- Frozen tissue extraction
- Ethanol-preserved sample extraction
Specialized Methods:
- Magnetic bead-based extraction
- Solid-phase extraction
- Filter-based extraction
- Alkaline extraction
- Modified Proteinase K digestion
Field-based Methods:
Room temperature preservation methods
Rapid extraction protocols
Mobile extraction kits
FTA cards
Protocol Optimization Strategies
Optimizing DNA extraction protocols is the next step after selecting a suitable technique for your wildlife samples. You’ll want to contemplate modifications to commercial kits, which can improve DNA yield and quality. A matrix-based separation protocol may enhance DNA collection from hard-to-lyse organisms, while techniques like grinding, bead-beating, and sonication can be used for mechanical disruption. Enzymatic pre-treatment can significantly improve extraction efficiency for commercial kits.
Don’t forget to remove enzyme inhibitors, as they’re essential for successful amplification. Specific protocols may be more suitable for certain sample types, such as iron incrustations and oilfield-produced water.
When adapting and customizing protocols, try combining upstream steps from one protocol with downstream steps from another. You can pause between steps for optimization and use a mix-and-match approach to tailor the protocol to your needs. Explore alternative kits and manual extraction methods for different sample types, and use electrophoretic analysis to assess the quality of extracted DNA.
Keep in mind that environmental DNA (eDNA) samples can introduce biases if not properly managed. Different environmental samples require specific considerations, like handling metal and salt contamination.
Inhibitor substances in complex matrices can interfere with PCR detection, and DNA fragmentation can occur in processed samples, affecting amplification. Remember, the effectiveness of nucleic acid extraction greatly influences target amplification success.
DNA Purification Methods
Purifying DNA is the next essential step after extraction, guaranteeing that your sample is free from contaminants and inhibitors.
You can choose from various physical, chemical, and column-based methods to achieve high-quality DNA for downstream applications.
Physical methods like centrifugation, filtration, precipitation, gel electrophoresis, and magnetic beads rely on the physical properties of DNA to separate it from other cellular components.
Chemical methods, such as phenol-chloroform extraction, ethanol precipitation, salting out, guanidinium thiocyanate, and cesium chloride density gradient, employ specific chemicals to isolate DNA from proteins and contaminants. Phenol-chloroform extraction, a traditional method, uses toxic reagents and requires careful handling.
Column-based methods, including silica columns, ion exchange columns, affinity chromatography, gel filtration, and spin columns, utilize different principles to purify DNA efficiently.
These methods involve binding DNA to a specific matrix, washing away impurities, and eluting the purified DNA.
Commercial kits, such as QIAGEN DNeasy Blood & Tissue Kit, BIOKÈ NucleoSpin Tissue Kit, Invisorb Spin Tissue Mini, NucleoSpin Food, and ZymoBIOMICS DNA Miniprep, offer standardized protocols and optimized reagents for DNA purification from various sample types.
When selecting a purification method, consider factors such as sample type, downstream applications, time, cost, and available resources.
Downstream Applications
Now that you’ve successfully extracted and purified DNA from your wildlife samples, it’s time to explore the fascinating world of downstream applications. DNA sequencing and microsatellite analyses enable you to identify species, differentiate individuals, determine sex, evaluate relatedness and paternity, and trace geographic origins.
You can harness these genetic analysis techniques for conservation management, estimating population sizes, analyzing communities, monitoring endangered species, evaluating diet and health, and conducting forensic investigations. Non-invasive DNA techniques allow you to study species without capturing or disturbing them, making it an invaluable tool for wildlife research.
Dive into the domain of molecular ecology, where mitochondrial DNA sequencing unravels species identification and phylogenetic relationships. Microbiome characterization provides insights into the microbial communities associated with wildlife, while pathogen diagnosis helps identify diseases. Evaluating genetic diversity within and among populations sheds light on evolutionary processes shaping wildlife.
In the field of ecological research, eDNA opens doors to revealing community composition and mapping species distributions. You can investigate ecological interactions, detect invasive species, and analyze habitat use.
By applying these downstream applications, you’ll contribute to a deeper understanding of wildlife biology, conservation, and management.
As you begin this journey, remember that the quality of your extracted DNA is vital for the success of downstream analyses. Adhere to best practices, maintain sterility, and optimize your protocols to guarantee reliable results.
With the power of DNA at your fingertips, you’re ready to unravel the mysteries of wildlife and make meaningful contributions to the field. So, let your curiosity guide you, and embrace the exciting possibilities that await in the world of wildlife DNA analysis.
Field Collection Techniques
Collecting wildlife samples in the field is a critical first step in the DNA extraction process. You can employ various sampling strategies, such as quadrat sampling for small-scale biodiversity assessments, transect sampling for broader spatial coverage, and non-invasive sampling using faeces, hair, or saliva. Quadrats and transects are ecological sampling techniques for effective sampling.
When collecting plant material, use young, fresh leaves or buds for better DNA yield. For animals, collect muscle tissue from foods like fish or red meat, or use internal organs and bone marrow. Obtain fruit bodies for fungal samples when possible. Environmental sampling can reveal unknown species diversity and distinguish genetic differences between similar-looking animals.
Consider ethical and legal aspects when collecting samples. Respect private property, avoid collecting endangered species, and minimize damage to living plants. Document the location using smartphone apps, especially for collaborative projects.
Devices like WaxTags can collect saliva DNA from small mammals but require optimization for different species. Protect samples from environmental degradation during preservation. Freeze samples at -20°C or below, or store them in a high-salt solution like DMSO. Use cold packs and insulated containers for short-term storage and transportation.
Proper sample collection and preservation are essential for successful DNA extraction and downstream applications.
Commercial Kit Comparison
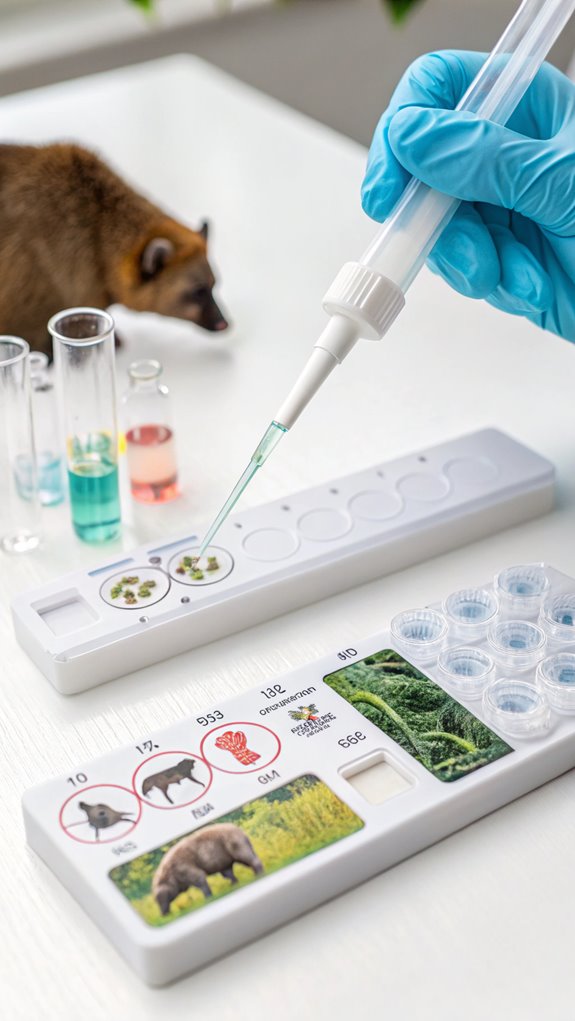
Selecting the right commercial DNA extraction kit is essential for optimizing your wildlife genomics workflow. Commercial kits offer advantages in time efficiency, DNA yield and purity, scalability, and quality assurance compared to traditional methods. Cluster analysis of different kits can reveal their impact on microbial diversity metrics.
Kits like the MagSi-DNA Animal Kit are suitable for cost-effective extraction from various animal samples, while the TIANamp Marine Animals DNA Kit is specifically developed for marine organisms. For fecal samples, consider the ISOLATE Fecal DNA Kit or PSP Spin Stool DNA Kit, and for soil samples, the FastDNA Spin Kit for Soil is optimized.
When choosing a kit, keep in mind the limitations and considerations. Kits can be more expensive and less customizable than traditional methods, and they’re optimized for specific sample types. Some kits include an ethanol removal step, while others like MagSi-DNA eliminate it.
Experimental validation and reproducibility are vital factors. Kits have been validated for applications like 16S rRNA gene amplicon sequencing and genotyping assays, ensuring consistent performance and reliability. They accommodate different scales of extraction and undergo rigorous quality control measures.
Quality Control Measures
Quality control is paramount in wildlife DNA extraction to guarantee reliable and accurate results. You must maintain detailed chain-of-custody records, implement quality assurance programs, use standard operating procedures, validate your lab, and conduct regular audits and proficiency tests.
In the lab, always include negative controls to detect contamination, analyze field and lab blanks to identify contamination sources, validate new assays through rigorous testing, monitor procedures with quality control standards, and document any issues that arise. Approximately 25% of eDNA studies published between 2008-2020 reported negative controls, with 95% lacking essential methodological information for reproducibility.
Proper sample handling and storage are also critical. Keep samples frozen until DNA extraction, avoid repeated thawing and refreezing, handle samples to minimize contamination risk, use single-use supplies, and maintain a controlled processing environment.
When interpreting and reporting data, standardize procedures, establish clear guidelines, include quality assurance measures, maintain transparency by providing detailed methods and results, and properly interpret negative control results.
Species-Specific Considerations
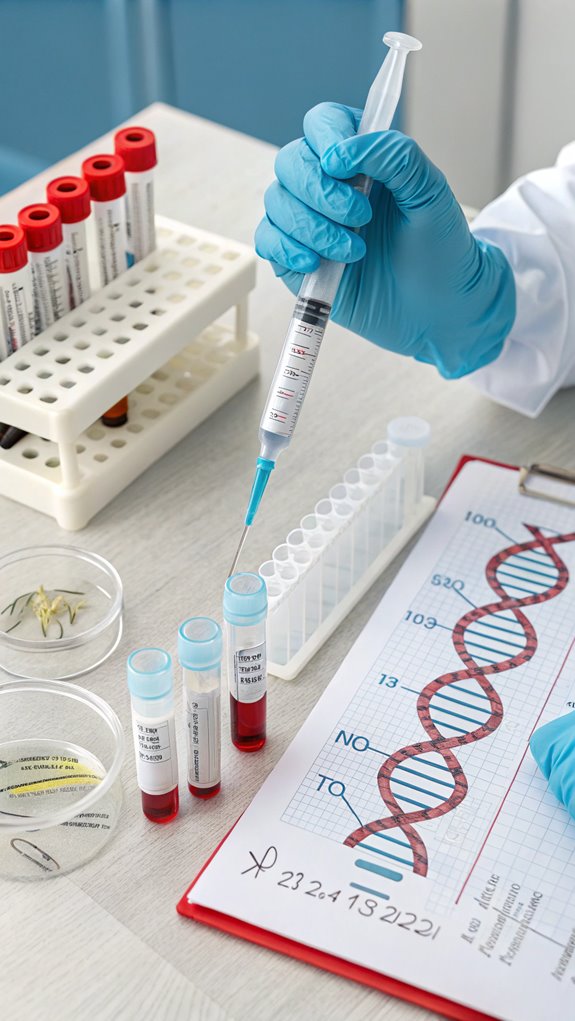
When extracting DNA from wildlife samples, you must take into account the unique challenges posed by different species. Variability in DNA markers means you’ll need to select appropriate genetic markers for each species. You should also guarantee there’s adequate reference data from various source populations, especially for determining geographic origin. DNA analysis of African ivory has identified poaching hotspots, enabling targeted conservation efforts.
Keep in mind that rare or nearly extinct species may have insufficient reference data, hindering DNA analysis.
You can extract DNA from a wide range of biological materials, including blood, meat, urine, faeces, skin, hair, scales, bone, feathers, claws, teeth, and processed products. However, highly processed products will require specialized DNA extraction methods.
For aquatic species, you may use environmental DNA (eDNA) from water samples, but be aware of sensitivity and specificity issues related to cross-contamination and primer design.
Developing species-specific assays is vital, and you should validate them for each target species. Quantitative PCR (qPCR) can provide both qualitative and quantitative information on species presence, while metabarcoding allows for the analysis of complex multi-species samples.
eDNA protocols must be refined for each species and environment to guarantee accurate detection.
In wildlife trafficking investigations, you’ll commonly use DNA testing for species identification, geographic origin determination, and individual identification.
DNA can also authenticate legal wildlife products and distinguish them from illegal ones. Standardizing DNA extraction and analysis methods is imperative for reliable results and forensic applications in prosecuting wildlife trafficking cases.
Troubleshooting Tips
Troubleshooting is an essential skill when extracting DNA from wildlife samples. If you’re facing insufficient DNA yield, consider factors like inadequate cell lysis, enzyme inactivity, inappropriate handling, high DNase activity, and column overload. JavaScript frameworks can help optimize protocols and workflows.
Verify you’ve used enough cells, prepared enzymes correctly, handled samples gently, and haven’t exceeded the column’s capacity.
DNA degradation can occur due to improper sample storage, high DNase content, incomplete lysis, using old blood samples, or tissue fibers clogging the membrane.
Store samples properly, verify complete digestion, and use fresh blood to minimize degradation.
Contamination issues like proteins, salts, RNA, incomplete tissue digestion, and ethanol can affect your results.
Verify complete digestion, remove guanidine salts and ethanol, and adjust input material and lysis time to avoid contamination.
Proper optimization and handling are vital.
Homogenize tissues completely, use ethanol correctly, maintain proper buffer ratios, elute DNA thoroughly with prewarmed TE buffer, and control water bath temperatures.

Erzsebet Frey (Eli Frey) is an ecologist and online entrepreneur with a Master of Science in Ecology from the University of Belgrade. Originally from Serbia, she has lived in Sri Lanka since 2017. Eli has worked internationally in countries like Oman, Brazil, Germany, and Sri Lanka. In 2018, she expanded into SEO and blogging, completing courses from UC Davis and Edinburgh. Eli has founded multiple websites focused on biology, ecology, environmental science, sustainable and simple living, and outdoor activities. She enjoys creating nature and simple living videos on YouTube and participates in speleology, diving, and hiking.
🌿 Explore the Wild Side!
Discover eBooks, guides, templates and stylish wildlife-themed T-shirts, notebooks, scrunchies, bandanas, and tote bags. Perfect for nature lovers and wildlife enthusiasts!
Visit My Shop →